The radula is a structure that is exclusive to molluscs with bivalves being the only class in the phylum that do not posses a radula as it has been secondarily lost. A radula is defined as a "flexible, longitudinal ribbon of transverse rows of tiny chitinous teeth" (Ruppert et al 2004) and is a feeding apparatus, used to scrape and cut food. The mouth of polyplacophorans opens up to a chitin-lined buccal cavity (mouth cavity) and a radular sac projects posteriorly from this, which houses the radula (Ruppert et al 2004). The construction of the radula is highly conserved and similar across all polyplacophorans as they all have 17 teeth in one transverse row, however there is huge variability in the number of transverse rows but this is usually minimal within a genus (Kaas et al 1998, Saito 2004). The individual teeth morphology of the radula within the polyplacophorans is highly variable and useful to distinguish species and also has also been found to be useful to determine phylogenetic relationship between the different taxa within the phylum (Kaas et al 1998, Saito 2004). One transverse row of teeth has two symmetrical halves, each with eight different teeth types and a central tooth, making 17 teeth all up (Figure 1).
Figure 3: SEM image of the centre of
Acanthochitona sp. radula, demonstrating the central and central-lateral teeth.
The major uncinus tooth, protruding laterally from the radula is tall, spoon-like but fairly thin (Figure 4). The most distinctive tooth is the major lateral tooth (Schwabe 2010) and it is also the most dominant scraping tooth of the radula (Saito 2004).
Acanthochitona sp. major lateral tooth has a long shaft that is fairly thin that ends in a large head, which has three distinct points and therefore is tridentate (Figure 4). A tridentate head on the major lateral tooth is a feature that is common in species within the Acanthochitonidae family (Kaas et al 1998). The head is flat anteriorly with fairly wide denticles (distinct points) with the two outer denticles having the same length but the middle denticle is slightly longer (Figure 4).
Figure 4: SEM image of Acanthochitona sp. radula, demonstrating the major lateral and major uncinus teeth.
Acanthochitona sp. major lateral head is black, as it is capped in an iron-containing material known as magnetite (Kaas et al 1998, Saito 2004, Ruppert et al 2004). Polyplacophoran radulas grown anteriorly, continuously, with the newest teeth being formed by odotophore cells found at the posterior end of the radula and the oldest teeth occur at the anterior end, which wear down over time and are replaced (Kaas et al 1998, Shaw et al 2008, Shaw et al 2010). A process of biomineralization occurs; where the new teeth become mineralised from the overlying epithelial tissue and both the teeth and epithelium tissue move forward as new teeth emerge (Shaw et al 2008, Shaw et al 2010). Canals in the major lateral tooth (Figure 1) are used to mineralise the teeth also (Shaw et al 2009). These pores were not seen on
Acanthochitona sp. and further investigation would be needed to determine whether they are present or not. Mineralization reinforces and hardens the teeth, making them durable and increasing their life span (Ruppert et al 2004, Shaw et al 2010). The process of biomineralization was observed in
Acanthochitona sp. radula as the anterior end was brightly coloured orange with black heads of the lateral teeth, demonstrating the oldest teeth, while the posterior end had lightly coloured new teeth. The implementation of minerals such as magnetites is said to be an adaptation of the radula, which allows polyplacophorans, such as
Acanthochitona sp. to graze upon algae on hard substrates (Shaw et al 2009, Ruppert et al 2004). For more information on the feeding mechanisms of
Acanthochitona sp. see
Feeding.
Digestive system
All polyplacophorans have a large body cavity where numerous organs and a three stage digestive tract (foregut, midgut and hind gut) occurs (Figure 5). The start of the digestive system in
Acanthochitona sp., like all polyplacophorans, involves the buccal cavity and radula. For information of the anatomy and a description of this area, see "Radula" above or visit the
Feeding page. Digestion in polyplacophorans is extracellular (break down of food by enzymes) and occurs in the digestive cecum, intestine and in the stomach (Ruppert et al 2004). After food scrapings are in the buccal cavity, mucous is released to form a string of food that is moved to the esophagus using cilia. The oesophagus has an anterior and posterior with sugar glands that start digestion of the passing food that moves into the stomach (Fretter 1937). The stomach has a large sac followed by a constriction in the middle leading to the anterior intestine (Fretter 1937). The digestive cecum releases enzymes into the stomach to digest food (Ruppert et al 2004, Kaas et al 1998). The stomach of species in the order Acanthochitonina have a wide sac that is found between lobes of the digestive gland (Kaas et al 1998). The anterior intestine is U shaped and the posterior is coiled and where faeces occur, with a valve between them (Fretter 1937). This is followed by the anus, where faeces are expelled and are released into the water column by the exhalant current.
Acanthochitona crintus, has a very similar digestive system to the classic polyplacophoran digestive system, described by
Lepidochitona cinerea (Fretter 1937). Only small differences were noticed such as
A. crintus has a larger stomach and the dorsal channel near the liver increases instead of decreases, but the coiling of the intestine is very similar (Fretter 1937).
Digestive system of a polyplacophoran.
A= Mouth.
B=Salivary gland.
C=Buccal cavity.
D= Esophagus.
E= Left esophagus gland.
F= Stomach.
G= Digestive cecum.
H= Anterior intestine.
I= Posterior intestine.
J= Anus. Adapted from Ruppert et al 2004.
Hemal system
In the coelom of all polyplacophorans, a heart is present which is essential for circulation around the body. Polyplacohporans have a large amount of hemolymph, which is the fluid found in their open circulatory system and this is usually colourless (Kaas et al 1998). The heart is found dorsally, towards to posterior, under the 7th and 8th valves and consists of a ventricle and and a pair of lateral atria (Figure 7). Blood enters the heart from the gills via atria, which moves the blood to the ventricle and then the blood makes its way to the hemocoel by the use of surrounding muscles (Ruppert et al 1998). An aorta is found dorsally, between the gonad and the top of the body cavity and this supplies blood to the gonad and musculature through many small channels (Kaas et al 1998). The heart appears to be similar across most polyplacophorans, but more research is needed to determine the exact structure of the heart found in Acanthochitona species.
Reproductive system
As mentioned in
Reproduction,
Acanthochitona sp. is dieoceous and have have both male and female sexes.
Acanthochitona sp. is a broadcast spawner and has external fertilisation, therefore no copulatory organs are present in the reproductive system.
Acanthochitona sp. has a paired gonad that is fused as only one genus, Nuttallochiton, has an unpaired gonad (Kaas et al 1998). The gonad is found found between the digestive tract and the dorsal surface of the body cavity and houses either sperm or eggs depending on the sex of the chiton (Figure 7). This gonad leads to paired gonopores, that are found on the left and right sides of the foot, in the pallial groove, between the second and third gill from the posterior (Yonge 1939). Eggs and sperm are excreted through these pores and are moved out into the water column by the exhalant current and out the exhalant aperture (Yonge 1939).
Nervous system
The nervous system of polyplacophorans is simple and they lack a cerebral ganglia. They do possess a buccal ganglia in the anterior head region and also a subradula ganglia, but these ganglia are not associated with nerves (Kaas et al 1998).
Acanthochitona species have a lateral nerve cord that surrounds the posterior part of the body and connected to the gills in the papillae groove (Valentine 2004, Kaas et al 1998)(Figure 6). Two pedal nerve cords run posteriorly in the centre of the body, dorsally in
Acanthochitona species foot. These are connected by horizontal pedal commissures and together with the pedal nerve cord, these connect to the complex musculature in the foot (Kaas et al 1998, Valentine 2004). The cerebrobuccal ring lines the head region and two buccal ganglia occur inside just above the buccul cavity musculature, followed by the buccal connective, subradula connectives and the two subradula ganglia found above the subradula organ (Valentine 2004). The dorsal buccal commissures have nerves that move the mouth and buccal cavity, whereas the ventral buccal commissure encircles the oesophagus and its nerves are used to move the radular (Kaas et al 1998, Valentine 2004).
Sense organs, known as osphradia are found in most polyplacophorans. In
Acanthochitona crinitus, they were found in the papillae groove, extending from the anus to the post renal gills. They consisted of a ciliated epithelium with sense cells that lead to the pallial nervous cord through nerve fibres (Yonge 1939). This is likely what is also seen in
Acanthochitona sp.
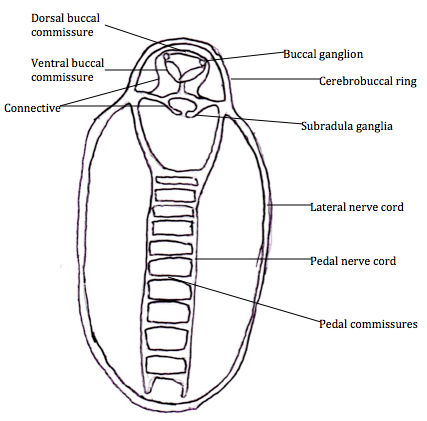
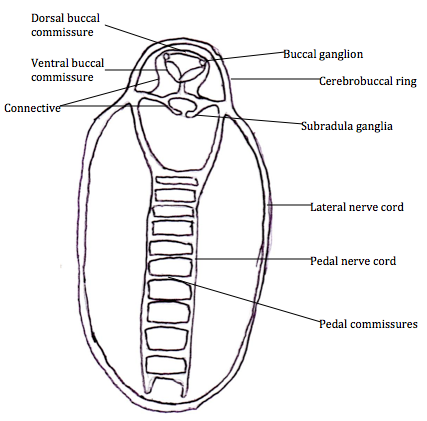 Figure 6:
Figure 6: The nervous system of
Acanthochitona, adapted from Valentine 2004.
Excretory system
The excretory system in Acanthochitona sp., is most likely similar to that observed in many plolyplacophorans (Figure 7). Two metanephridia are found ventrally and these remove metabolic wastes that are released through the nephridiopore, which is found ventrally, on either side of the pallial groove near the posterior end, near the post-renal gills (Yonge 1939). The excretory system begins with a renopericardial canal, with a renopericardial pore, leading to the nephirdial canal and then the nephridiopore (Kaas et al 1998). Ultrafiltration of hemolymph occurs in the renopericardial canal to create primary urine, which makes its way to the nephridial canal where further excretion and reabsorption occur to create the final urine that leaves through the nephridiopore (Kaas et al 1998, Ruppert et al 2004). The nephridial canal has many outpouching areas known as diverticula and also has a ciliated epithelium to increase surface area and to facilitate the excretory and reabsorption process (Kaas et al 1998). This system is conserved across all polyplacophorans, just the complexity of the system changes across different groups.
Musculature
Polyplacophorans have complex musculature but this enables then to be very flexible, mover over almost any substrates and attach themselves firmly. The rectus muscles are two muscles that run from the anterior region to the posterior in the middle of the animal, in the foot, and attaches to the ventral surface of the valves (Kaas et al 1998, Sampson 1895). This enables the chiton to stretch out. Diagonal oblique muscles occur laterally to the rectus muscle and also attach to each of the valves. Each valve is connect to each other by transverse muscles that attaches the sutural laminae which occurs anteriorly, to the posterior of the preceding valve, allowing the articulated valves to be flexible and manouverable. The lateral longitudinal muscle occurs around the whole chiton, just under the periphery of the valves and are important in a chitons defence mechanism. Finally, two latero-pedal muscles attach laterally on each valve, consisting of an anterior latero-pedal muscle and a posterior later-pedal muscle, which connect the valves to the muscular foot (Kaas et al 1998).
The girdle also consists of many fibres that run longitudinally and horizontally that can clamp and flex and are also associated with the gills and the gill lamellae (Sampson 1895, Kaas et al 1998). Broad flat muscles and thin muscles attach from the radula sheath to the muscle attachment area of the radula know as the "cartilages". Protractor muscles attached to the first valve, attach to the broad muscles on the muscular sheath and protractor muscles also attach, allowing movement of the radula (Sampson 1895). For more information, see
Behaviour and Locomotion.